
Quick transfer taking anywhere between 5 and 30 minutes.The specialist transfer machine will then apply a current through the cassette to initiate transfer. Transfer buffer is used however the components of the sandwich are soaked in the transfer the buffer instead of being surrounded by it. Semi-dry transfer uses the same principles as wet-transfer but instead the membrane lays horizontally in a cassette instead of vertically in a tank. Requires large amounts of buffer and methanol.Longer Transfer Time (1h/2h or even overnight).Good for high molecular weight proteins and low abundant proteins.The gel and membrane are placed in a traditional transfer sandwich which comprises of filter paper and sponge pads. The tank is filled with transfer buffer, which is similar to running buffer as it allows for the facilitation of the electrical current, but also contains methanol which allows for the adsorption of proteins onto the membrane. In wet transfer a similar gel tank is used to SDS-PAGE in that the gel and membrane stand vertically, but this time with the current moving across instead of down the tank. We strongly recommend PVDF membranes (or PSQ membranes with 0.22um micropores for targets less than <30 kDa).Īlthough all transfer techniques use the same basic principle of transferring proteins onto a membrane using an electric current, there are several different methods of western blot transfer: 1. There are different types of membranes to choose from, the most popular being PVDF or nitrocellulose. This membrane can then be probed for proteins of interest using primary antibodies. This is achieved by an electric current again, causing the negatively charged proteins to move out of the acrylamide matrix and onto a specified western blot membrane. The transfer step simply transfers these proteins from the acrylamide gel matrix onto a membrane. Use gel-loading pipette tips to facilitate easy and even loading of your protein samples.
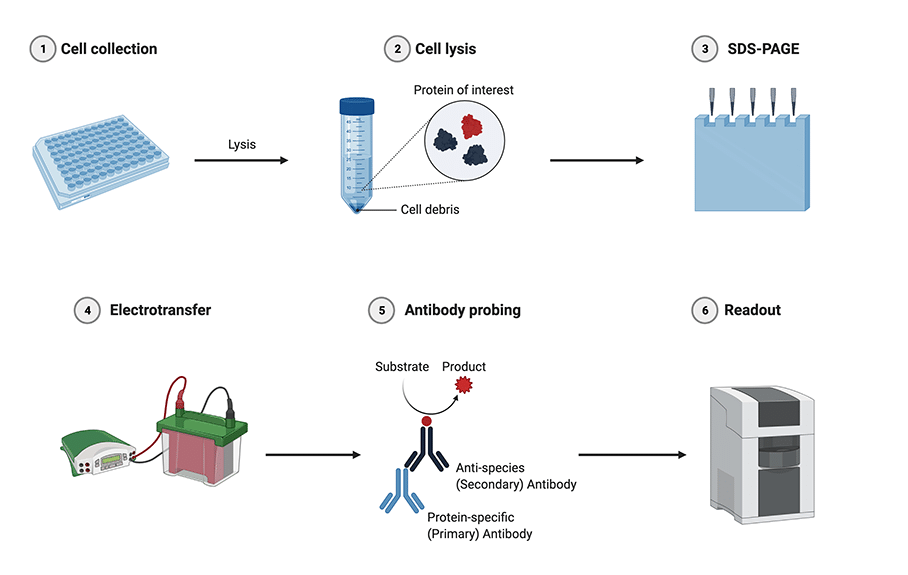
After removing gel combs rinse each well thoroughly with running buffer to ensure no remaining bits of acrylamide are blocking them. (<20kDa) better than Tris-glycine gels.įigure 1. Tip: Tris-tricine gels separate low MW proteins. Stop the gel running when the dye front migrates to the desired position. Turn on electrophoresis power pack and set to a low voltage (as the sample runs through the stacking gel), increasing to a higher voltage (e.g., 120V) when the dye front reaches the separating layer. Load samples and appropriate protein markers onto the gel using a tip. Remove gel combs and cleanse wells of any residual stacking gel by pipetting running buffer up and down in each well using gel-loading tip (Figure 1). Set up electrophoresis apparatus and immerse in 1X running buffer. Add 4X SDS sample buffer so the total protein amount is 30 - 50ug per sample (according to the protein amount measured by Bradford or BCA protein assay).įlick microfuge tubes to mix samples, spin them shortly, and then heat to 95 - 100 ℃ for 5 minutes. (For recipes see the " SDS-PAGE gel recipes" section) The end result is an acrylamide gel containing all the proteins in your lysate separated due to size and charge.Ĭonstruct an SDS-PAGE gel according to the molecular weight (MW) of your target protein(s). Smaller proteins migrate faster, and larger proteins migrate slower. This allows the linearized negatively charged proteins to migrate towards the positive electrode at different speeds through the matrix, depending on their size. In PAGE (Polyacrylamide gel electrophoresis) an electric current is applied to an acrylamide matrix. When exposed to an electric current, these negatively charged linear proteins will be attracted to the positive electrode. As a strong detergent, SDS not only linearizes but also adds a negative charge to the elongated proteins. This is achieved by treating the lysate prepared previously with loading buffer that contains SDS (Sodium Dodecyl Sulfate). SDS-PAGE is the process by which proteins are denatured and linearized, before being separated according to their molecular weight. The overall principle of western blot workflow includes 4 main steps: separation by size, transfer to membrane, immunoblotting with antibodies and visualization of your probed proteins. Once the lysate has been prepared and protein concentration determined the main western blot workflow can begin. This allows the researcher to determine a number of things about their sample including what proteins are present and at what level are they expressed, as well as possible post-translational modifications such as phosphorylation. This sample is usually called a lysate, which is the product of lysing cells or tissues to release all the protein contents within that cell type or tissue. Western blot is an analytical technique used to detect and determine the abundance of specific proteins of interest within a sample.


 0 kommentar(er)
0 kommentar(er)
